Virpax Pharmaceuticals, Inc. is a preclinical stage biopharmaceutical company, which engages in the development of branded, non-addictive pain management products. Its products include metered-dose spray, liposomal gel encapsulates, and intranasal spray.
On Tuesday August 17th Virpax Pharmaceuticals announced that they have received FDA Response and Guidance on MMS019 drug.
We are very pleased with the response from the FDA. We believe that the initial pathway to move forward with the development of MMS019 has been clarified. The pre-IND meeting provides an opportunity for open communication between the Sponsor and the FDA to discuss the IND development plan and to obtain the FDA’s guidance for clinical studies for the new drug candidate. As our development program proceeds, we will define the strategy for our drug-device combination product candidate, MMS019, for use in an over-the-counter setting as we look to support a consumer-friendly OTC indication.”
Anthony P. Mack – Chairman and CEO of Virpax Pharmaceuticals
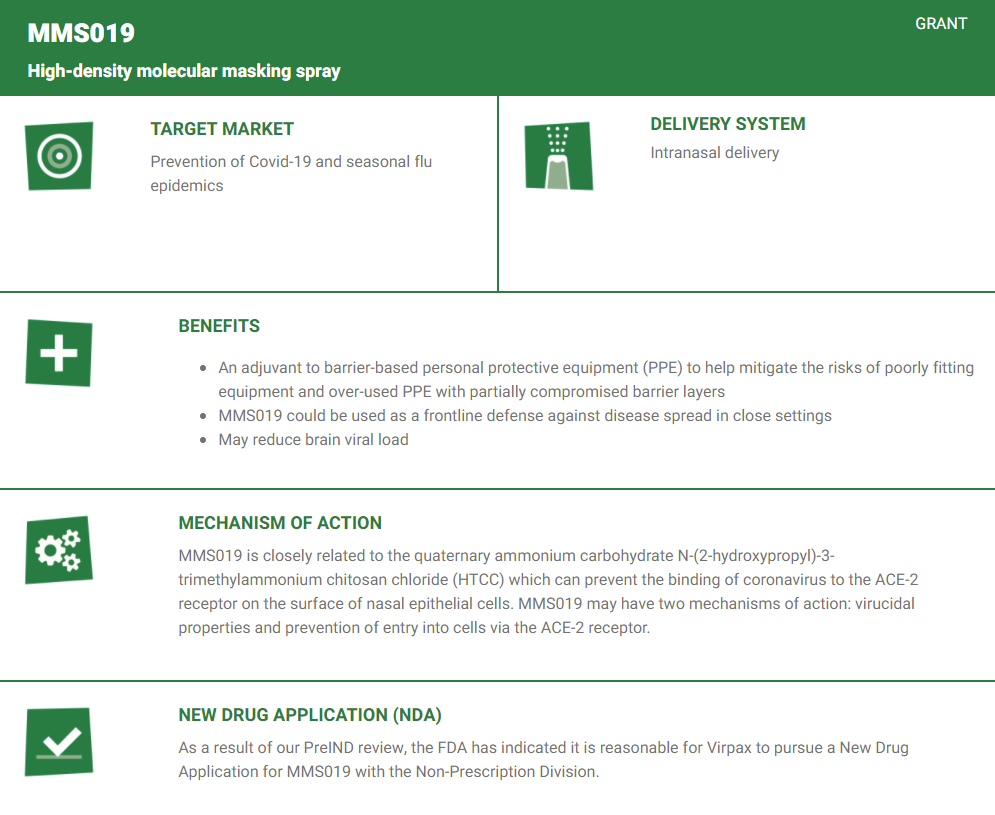
What is MMS019?
MMS019 is a patented and proprietary high-density molecular masking spray under development for use as an anti-viral barrier product. MMS019 is a drug product candidate based on a type of nanotechnology that enables the exclusive delivery of a metabolically labile peptide drug into the brain on intranasal delivery. MMS019 is manufactured using industrially relevant equipment and processes (high pressure homogenization and spray drying). There is pharmacological evidence of activity of molecular envelope technology (MET) enabled enkephalin in morphine-tolerant animals. The MET nanoparticles are well tolerated via the nasal route at the dose administered. MMS019 demonstrated comparable preclinical activity to morphine in all animal pain models tested without the drug seeking and tolerance associated with opioids.