Launch of novel cell and gene therapy by market players is expected to augment the market growth
Key players in the market are focused on launching novel cell and gene therapies in order to treat various diseases and address the critical unmet needs of patients. For instance, in June 2011, Fibrocell Technologies, Inc. received approval from the U.S. Food and Drug Administration (FDA) for Azficel-T, which can be used for the treatment of nasolabial fold wrinkles.
Moreover, in November 2011, New York Blood Center, Inc. received approval from the FDA for Hemacord. It is indicated for unrelated donor hematopoietic progenitor cell transplantation procedures and in patients with disorders affecting the hematopoietic system that are inherited, acquired, or results from myeloablative treatment in children. In October 2017, Kite Pharma received approval from the U.S. FDA for Yescarta (axicabtagene ciloleucel) which is used for the treatment of adults with large B-cell lymphoma. It was the first chimeric antigen receptor (CAR) T-cell therapy to receive FDA approval for large B-cell lymphoma.
Get PDF Brochure of Research Report: https://www.coherentmarketinsights.com/insight/request-pdf/2475
Browse 35 Market Data Tables and 28 Figures spread through 164 Pages and in-depth TOC on Cell and Gene Therapy Market, by Therapy Type (Cell Therapy and Gene Therapy), by Indication (Cardiovascular Disease, Cancer, Genetic Disorders, Infectious Diseases, Neurological Disorders, and Others), by Scale of Operation (In-house and Outsourced), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) – Global Forecast to 2026.
Key players in the market are focused on adopting strategies such as mergers and acquisitions to enhance their product portfolio. For instance, in June 2017, Johnson & Johnson acquired Actelion Ltd. Moreover, in October 2017, Gilead Sciences acquired Kite Pharma. Kite Pharma is involved in developing chimeric antigen receptor T-cell therapy, to recognize and attack malignant cells.
Key Takeaways of the Cell and Gene Therapy Market:
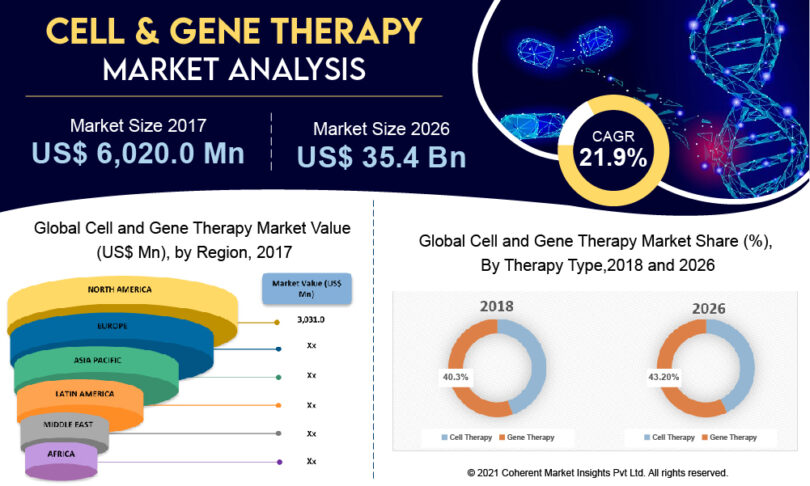
• The global cell and gene therapy market is expected to exhibit a CAGR of 21.9% during the forecast period owing to the increasing prevalence of cardiovascular diseases, cancer, and neurological disorders.
• Among therapy type, the gene therapy segment held a dominant position in the cell and gene therapy market in 2018. For instance, in November 2018, Pharma Intelligence Company estimated that around 55% of the pipeline is of gene therapy, and medicines that are delivered in vivo.
• Among indication, the cancer segment held a dominant position in the cell and gene therapy market in 2018, as majority of cell and gene therapies consist of cancer medicines in development stage.
• Key players operating in the global cell and gene therapy market are Amgen, Biogen, BioMarin Pharmaceuticals, Bristol-Myers Squibb Company, GlaxoSmithKline, Novartis, Pfizer, Regeneron Pharmaceuticals and Sanofi, Spark Therapeutics, Agilis Biotherapeutics, Angionetics AVROBIO, Freeline Therapeutics, Horama, MeiraGTx, Myonexus Therapeutics, Nightstar Therapeutics, Kolon TissueGene, Inc., JCR Pharmaceuticals Co., Ltd., and MEDIPOST.
Buy-Now this research report: https://www.coherentmarketinsights.com/insight/buy-now/2475
Contact:
Coherent Market Insights
1001 4th Ave, #3200 Seattle, WA 98154, U.S.
Email: sales@coherentmarketinsights.com
United States of America: +1-206-701-6702
United Kingdom: +44-020-8133-4027
Japan: +050-5539-1737
India: +91-848-285-0837