𝐆𝐥𝐨𝐛𝐚𝐥 𝐑𝐞𝐬𝐩𝐢𝐫𝐚𝐭𝐨𝐫𝐲 𝐃𝐞𝐯𝐢𝐜𝐞𝐬 𝐌𝐚𝐫𝐤𝐞𝐭
𝐎𝐯𝐞𝐫𝐯𝐢𝐞𝐰
Some of the most often used respiratory devices are inhalers, nebulizer treatments, oxygen reflectors, positive airway pressure (PAP) devices, motorized ventilators, pulse oximeters, polysomnography devices, and spirometers.
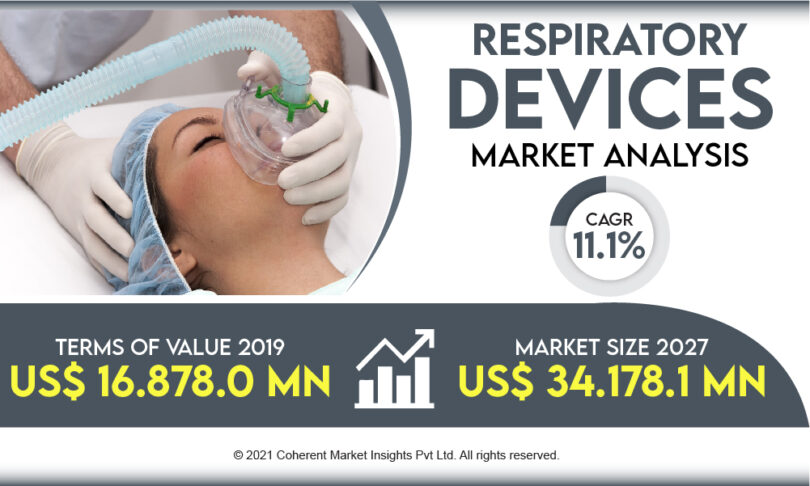
The global respiratory devices market is anticipated to reach US$ 34,178.1 million by the end of 2027, with a value of US$ 16,878.0 million in 2019.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐟𝐨𝐫 𝐒𝐚𝐦𝐩𝐥𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐚𝐭 –https://www.coherentmarketinsights.com/insight/request-sample/1299
𝐃𝐫𝐢𝐯𝐞𝐫𝐬
During the forecast period, rising air pollution is projected to drive the global respiratory devices market’s development. Air pollution (PM2.5, ozone, and home air pollution) is the sixth largest cause of death globally, according to the Health Effects Institute’s State of Global Air 2019 study.
𝐎𝐩𝐩𝐨𝐫𝐭𝐮𝐧𝐢𝐭𝐢𝐞𝐬
Players in the global respiratory devices market may anticipate attractive development possibilities if they invest and fund R&D for breathing problems. Peter Weiss AO, an Australian businessman and arts patron, committed US$ 4 million in support for essential lung disease research in February 2020.
𝐑𝐞𝐬𝐭𝐫𝐚𝐢𝐧𝐭𝐬
Critical mistakes in inhalation testing are common in respiratory devices, which is anticipated to stymie market expansion. Inhaler handling errors can have a negative influence on medication delivery and treatment effectiveness. Lack of drug adherence is also estimated to restrict market expansion. When compared to other chronic illnesses, asthma and COPD have poorer adherence rates.
𝐊𝐞𝐲 𝐓𝐚𝐤𝐞𝐚𝐰𝐚𝐲𝐬
The positive airway pressure devices segment of the global respiratory devices market was worth US$ 4,551.5 million in 2018 and is projected to grow at a CAGR of 11.1 percent to US$ 11,814.6 million by 2027. During the forecast period, the segment’s growth will continue to be aided by an increase in product releases.
The portable oxygen concentrators sub segment held the leading position in the oxygen concentrators segment in the global respiratory devices market in 2018, contributing for 66.6 percent share in terms of value, preceded by home oxygen concentrators. Future product releases are anticipated to drive the growth of this category.
In terms of value, the pulse oximeters sector accounted for 12.7 percent of the worldwide respiratory devices market. The pulse oximeters sub-segment is projected to rise as regulatory approvals for pulse oximeters increase. Masimo obtained FDA authorization for their O3 Regional Oximetry product’s newborn application in June 2019.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐇𝐞𝐫𝐞 𝐏𝐃𝐅 𝐁𝐫𝐨𝐜𝐡𝐮𝐫𝐞 @ https://www.coherentmarketinsights.com/insight/request-pdf/1299
𝐌𝐚𝐫𝐤𝐞𝐭 𝐓𝐫𝐞𝐧𝐝𝐬
The global respiratory devices market’s major players are concentrating on the development and introduction of new products. Pulmogine, a mesh nebulizer, was introduced at the 2019 ERS International Congress by HCmed Innovations Co., Inc., a drug-device combination business.
Product recalls are anticipated to stifle market expansion. Teleflex Incorporated issued a global voluntary recall of the COMFORT FLO Humidification System in February 2020, citing the risk of water flooding the section and entering the circuit if an unusual pressure drop is formed between the water bottle and the section all through high-flow oxygen therapy.
𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐢𝐨𝐧
𝐄𝐮𝐫𝐨𝐩𝐞
• Medical gas systems need to be CE Certified and Labelled in accordance with Council Directive 93/42 EEC Concerning Medical Devices (Class IIB); 2006/42 EC Machinery Directive; 97/23/EEC Pressure Equipment Directive (PED) and 2006/95/EC, Low Voltage Electrical Equipment Directive (LVD)
• In Europe, the MOS systems are treated as medical devices and classified as IIB
• They require a CE mark for legal marketing. However, the CE Marking process will change as Europe’s new Medical Device Regulation (MDR) comes into force in May 2020
Implementation, modification, and maintenance of a quality system (usually ISO 13485) that will meet European and other international requirements are further specifications
𝐏𝐮𝐫𝐜𝐡𝐚𝐬𝐞 𝐭𝐡𝐢𝐬 𝐏𝐫𝐞𝐦𝐢𝐮𝐦 𝐑𝐞𝐩𝐨𝐫𝐭 –https://www.coherentmarketinsights.com/insight/buy-now/1299
𝐂𝐨𝐦𝐩𝐞𝐭𝐢𝐭𝐢𝐯𝐞 𝐋𝐚𝐧𝐝𝐬𝐜𝐚𝐩𝐞
Key companies contributing in the global respiratory devices market are Hamilton Medical AG, ResMed. Inc., Fisher & Paykel Healthcare Limited, General Electric Healthcare Limited, Masimo Corporation, Inogen Inc., Teleflex Incorporated, Drägerwerk AG & Co. KGaA, Smiths Medical, Koninklijke Philips N.V., and Medtronic PLC.
𝐊𝐞𝐲 𝐃𝐞𝐯𝐞𝐥𝐨𝐩𝐦𝐞𝐧𝐭𝐬
June 2019: Rapid Oxygen collaborated with the Connecticut Alliance of Boys & Girls Clubs to donate its R15, a portable oxygen cylinder.
February 2020: Medtronic Pharmaceuticals stated that the firm and the Medtronic Foundation are donating about US$ 1.2 million to coronavirus disaster relief globally.
𝐂𝐨𝐧𝐭𝐚𝐜𝐭 𝐔𝐒:
Coherent Market Insights
1001 4th Ave, #3200 Seattle, WA 98154, U.S.
Email: sales@coherentmarketinsights.com
United States of America: +1-206-701-6702
United Kingdom: +44-020-8133-4027
Japan: +050-5539-1737
India: +91-848-285-0837