𝐆𝐥𝐨𝐛𝐚𝐥 𝐁𝐥𝐨𝐨𝐝 𝐂𝐥𝐨𝐭𝐭𝐢𝐧𝐠 𝐅𝐚𝐜𝐭𝐨𝐫𝐬 𝐌𝐚𝐫𝐤𝐞𝐭 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬
𝐎𝐯𝐞𝐫𝐯𝐢𝐞𝐰
Proteins or enzymes that regulate bleeding are known as blood clotting factors. Blood clotting, also known as blood coagulation, is a process in which blood is transformed into solid clots when a lesion or blood artery breaks down. Clotting can help avoid mortality from bleeding by preventing germs and viruses from entering the body. Hemostasis is the clotting process, which is regulated by a group of proteins known as blood clotting factors. Blood clotting disorders including hemophilia and von Willebrand disease can be caused by the scarcity of these factors (VWD). According to the Centers for Disease Control and Prevention (CDC), about 20,000 men in the U.S. had hemophilia in 2017.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐟𝐨𝐫 𝐒𝐚𝐦𝐩𝐥𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 𝐚𝐭 – https://www.coherentmarketinsights.com/insight/request-sample/1301
𝐃𝐫𝐢𝐯𝐞𝐫𝐬
During the forecast period, the licensing of new medicines is likely to drive the global blood clotting factors market growth. CSL Behring obtained confirmation from the Korea Ministry of Food and Drug Safety in January 2020 for Afstyla, a single-chain recombinant antihemophilic factor VIII therapy for patients with Hemophilia A. In addition, the European Commission granted Novo Nordisk marketing authorization for Esperoct in Europe in June 2019. Turoctocog alfa pegol, N8-GP, is a brand name for turoctocog alfa pegol, a drug used to treat hemophilia A in adolescents and adults (congenital factor VIII deficiency).
The global blood clotting factors is projected to expand due to the high frequency of hemophilia. Hemophilia A impacted 1 in every 5,000 male births in the U.S., according to statistics released by the Centers for Disease Control and Prevention (CDC) in September 2018. Likewise, according to Cleveland Clinic data from 2016, Hemophilia impacted 1 in every 25,000 to 30,000 boys.
Increasing efforts and contributions for helping the people treated with Hemophilia is projected to boost the market expansion during the forecast period. In April 2019, Grifols Worldwide SA, a producer of plasma derived products, provided more than 100 million international units of blood clotting factor medicines under the long-term goal of giving support to the Hemophilia sufferers.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐇𝐞𝐫𝐞 𝐏𝐃𝐅 𝐁𝐫𝐨𝐜𝐡𝐮𝐫𝐞 @ https://www.coherentmarketinsights.com/insight/request-pdf/1301
𝐑𝐞𝐠𝐢𝐨𝐧𝐚𝐥 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬
Hemophilia is a rare blood condition that affects just a tiny percentage of the world’s population. Due to the low return on investment, pharmaceutical companies invest less in the development of such medicines. The FDA, on the other hand, has acknowledged this fact and has developed a favorable policy to encourage businesses to invest in this field. The Orphan Drug Act, which applies to companies in this field, offers three special incentives: a seven-year exclusivity period, a tax credit, and the elimination of prescription drug user fees. As a result of this policy, the company has invested in rare disease research, leading to the development of products like Hemlibra (Emicizumab-kxwh), Adynovate, and others.
In advanced economies such as the U.S. and Canada, the soaring number of commercial authorizations and heightened recognition about rare diseases has resulted in a large number of patients receiving treatment. North America has dominated the market due to the factors mentioned above, and it is projected to continue to do so during the forecast period.
Due to an increase in the number of individuals diagnosed with chronic, advantageous compensation policies, and more product launches by market participants, Asia Pacific is projected to expand rapidly. Roche Products India Pvt. Ltd introduced Emicizumab, a hemophilia A medication marketed as Hemlibra, in India in April 2019. Through Factor VIII inhibitors, it is suggested as a preventive therapy for lowering the frequency of bleeding episodes in individuals with hemophilia A. Outpatient hemophilia therapy is supported by health insurance systems in more than 80 percent of Chinese cities, according to a 2012 study by the Chinese Ministry of Health, with compensation limitations and customer co-pay demands of more than 50%.
𝐂𝐨𝐦𝐩𝐞𝐭𝐢𝐭𝐢𝐯𝐞 𝐋𝐚𝐧𝐝𝐬𝐜𝐚𝐩𝐞
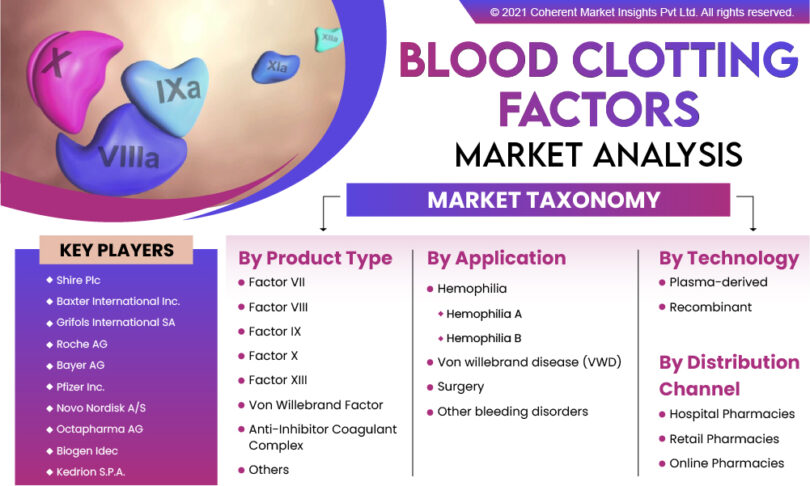
Major companies contributing in the global blood clotting factors market are Roche AG, Pfizer Inc., Grifols International SA, Baxter International Inc., Shire Plc, Kedrion S.P.A., Biogen Idec, Octapharma AG, Novo Nordisk A/S, and Bayer AG.
𝐏𝐮𝐫𝐜𝐡𝐚𝐬𝐞 𝐭𝐡𝐢𝐬 𝐏𝐫𝐞𝐦𝐢𝐮𝐦 𝐑𝐞𝐩𝐨𝐫𝐭 –https://www.coherentmarketinsights.com/insight/buy-now/1301
𝐂𝐨𝐧𝐭𝐚𝐜𝐭 𝐔𝐒:
Coherent Market Insights
1001 4th Ave, #3200 Seattle, WA 98154, U.S.
Email: sales@coherentmarketinsights.com
United States of America: +1-206-701-6702
United Kingdom: +44-020-8133-4027
Japan: +050-5539-1737
India: +91-848-285-0837